Steen Jakobsen & Anna Christina Schacht, April 2016
C-11 Methylation
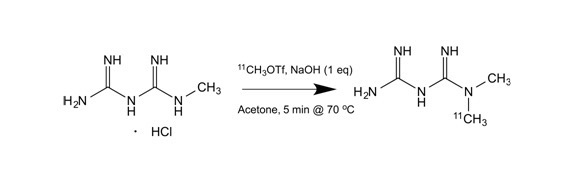
The most common isotope in PET tracers is carbon-11, incorporated into the molecule primarily by a methylation reaction. C-11 labelled carbon dioxide or methane delivered from the cyclotron is converted to C-11 methyl iodide or C-11 methyl triflate. A precursor molecule containing either an alcohol, amine or thiol functionality will react with the C-11 methylation reagent to yield the desired methylated tracer through nucleophilic substitution reactions. To date, more than 250 molecules have been labelled in this way. One example of a C-11 methylation reaction is depicted below in which the precursor, 1-methyl-biguanide, reacts with C-11 methyl triflate to give the desired tracer, C-11 metformin. Currently, C-11 metformin is used in three different research projects involving humans.
Multistep Methylation
Complex reaction schemes including 2-3 step reactions can only be accomplished with quick and efficient C-11 methylation reactions. An example is protected precursors; these are necessary to selectively label a molecule with multiple reaction sites. Herein, labelling of the precursor is followed by a deprotection step, which is time-consuming and lowers the overall yield of the final product.
Applying a combinatorial approach in the labelling strategy can be advantageous for creating a library of compounds used for preclinical research. First, a small (reporter) molecule is efficiently labelled with C-11 methyl, which can be reacted further with a range of compounds employing a specific type of reaction.
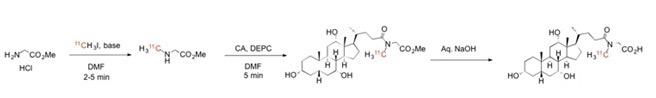
Ficture 2 shows the 3-step synthesis of C11 cholylsarcosine (Csar), a tracer that used in clinical examinations of humans?.
This methodology is further extended to taurine-conjugated bile acids and to date, five different taurine-containing tracers have been evaluated in preclinical studies.