PET studies of de novo Parkinson’s disease patients
Primary investigator:
Per Borghammer, Professor MD PhD DMSc
Investigators:
Karoline Knudsen , BMLT MMDI PhD
Jacob Horsager, PhD student
Katrine Andersen, PhD student
In recent years, involvement of the autonomic nervous system in Parkinson’s disease (PD) has gained substantial focus. Constipation is seen in >50% of patients up to 20 years prior to time of diagnosis, and disease specific pathology in the form of phosphorylated alphasynuclein (-syn) has been detected in the gastrointestinal (GI) tract in both prodromal and manifest PD. This has led to the Braak hypothesis proposing that PD pathology in some cases may initiate in autonomic GI nerve terminals and subsequently spread to brain structures via parasympathetic and sympathetic routes.
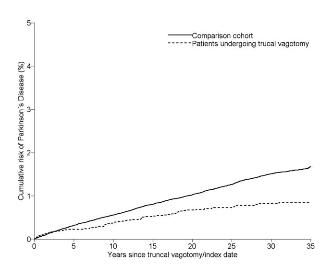
We have previously shown that patients undergoing full vagotomy, i.e. complete resection of the parasympathetic vagus nerve which innervates most of the gut organs and GI tract, have a 50% decreased risk of developing PD after 20 years of follow-up. This supports the hypothesis that PD may arise in the nerve terminals of the GI tract and spread through the vagus (Figure 1).
We have also shown that patients with idiopathic REM sleep behavior disorder (iRBD), now known to be a prodromal symptom of PD or dementia with Lewy Bodies (DLB), exhibit severely decreased parasympathetic and sympathetic innervation of peripheral organs (GI tract and heart) at the level of diagnosed moderate disease stage PD patients. In contrast, the brain dopamine system is only mildly affected in these patients (Figure 2, Figure 3). Thus, it seems that autonomic peripheral involvement is more pronounced at this early disease stage, supporting the aforementioned ”body-first” hypothesis. On the other hand, other studies have shown contradicting results. Therefore, it is theoretically possible that PD can be divided into at least two subgroups – one initiating in the GI autonomic nervous system, and one initiating in the central nervous system, in similarity to the subtypes of Creutzfeld-Jacob disease.
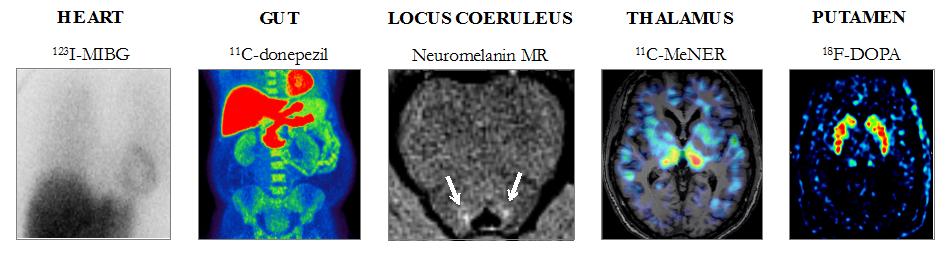
Figure 2. Heart sympathetic innervation measured by 123I-MIBG, small intestinal- and colon parasympathetic innervation measured by 11C-donepezil, pigmented neuronal density in the locus coeruleus measured by neuromelanin MR, thalamic noradrenergic innervation measured by 11C-MeNER, and putaminal dopamine storage capacity measured by 18F-DOPA in PD, iRBD, and HC subjects. Knudsen et al. Lancet Neurol, 2018.
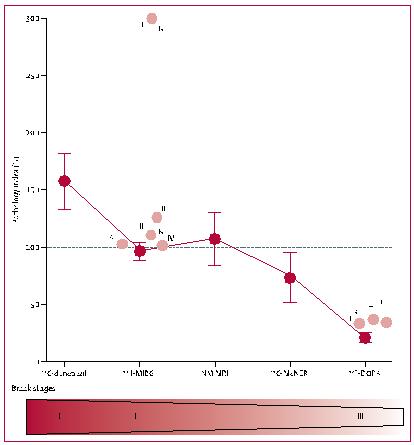
Figure 3. Patology-index (mean ±SEM). Mean for healthy controls (C) is defined as 0% and mean for diagnosed PD patients is defined as 100%. The figure shows that prodromale PD patients (iRBD patients) exhibit fully developed pathology at the level of diagnosed PD patients in the parasympathetic (DON) and sympathetic (MIBG) nervous system as well as in the noradrenergic nucleus locus coeruleus (NM) in the pons. On the contrary, the dopamine system (FDOPA) is still relatively spared at this stage.
This supports the hypothesis of PD pathology initiating in the periphery, subsequently spreading slowly to brain structures. Light red circles indicate pathology-index data calculated from 4 previous studies of iRBD patients (a-d) which supports the findings to be representative for iRBD patients in general. [DON: donepezil colon-values, MIBG: sympathetic innervation of the heart, NM: neuromelanin MRI signal, FDOPA: striatal dopaminergic innervation]. Knudsen et al. Lancet Neurol, 2018.
Only about half of PD patients have RBD and in some of these subjects, the symptom is present in the prodromal phase prior to diagnosis. Also, PD+RBD has been shown to be a more malignant phenotype comprising a more extensive range of symptoms and more rapid disease progression compared to PD-RBD.
In the present study, two groups of PD patients are included; PD+RBD and PD-RBD to examine a potential phenotypic difference. To document the exact disease difference without interference of potential treatment side effects like constipation and changes in microbial composition, only newly diagnosed patients, who have not yet started anti-parkinsonian treatment, are included. The participants undergo PET and SPECT scans of the peripheral sympathetic and parasympathetic nervous system as well as a comprehensive battery of GI function measures, intestinal permeability, microbial composition, and presence of intestinal -syn pro-aggregating bacterial toxins.
If we can show that PD+RBD patients exhibit more severe peripheral disease involvement and more pathological intestinal functional measures compared to PD-RBD, it will further support the hypothesis of several existing PD phenotypes. The study has the potential to change our fundamental understanding of PD, i.e. that the disease in some patients may initiate in the peripheral GI nervous system, potentially as a result of increased intestinal permeability and influence of pro-aggregating bacteria - and in other patients in the central nervous system.