Characterizing Tissue Microstructure by MR: The virtual biopsy
Our research group studies the relation between MR signals and tissue microstructure. Biopsies only sample a small fraction of the affected tissue. A biopsy in the brain is rarely performed because of the associated risks. Our long-term aim is to develop MR methods that are sensitive and specific to characteristic disease changes, which will increase the diagnostic and prognostic power of MRI.
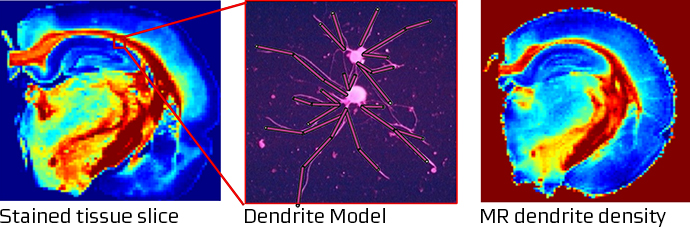
Our work involves several, integrated steps: First, we use our knowledge of magnetic resonance physics and statistical physics to build biophysical models that predict how specific microstructural features affect the MRI signal obtained by various pulse sequences. In our first research programme, we e.g. modeled how diffusing water molecules interact with neuron dendrites, and how individual neurites thereby affect the diffusion weighted MR signal. In the brain, the number of dendrites change during neurodevelopment and decline early in the development of depression and dementia. Noninvasive methods to determine dendrite density would thus be a major step forward in our understanding of brain disorders. Then, we fit the model to high quality MRI data, acquired for example on our 9.4 Tesla MR system and compare the model predictions to the ‘ground truth’ – in this case the density of dendrites as determined by state-of the art microscopy and stereological analysis. The figure illustrates how dendrites can be approximated by cylinders (middle panel). The effects of dendrites? on water diffusion can be modeled and derived from diffusion weighted MR images in a brain slice (right panel). To the right, dendrites and axons are stained, and by meticulously counting them, the MR method predictions could be verified (Jespersen 2010).
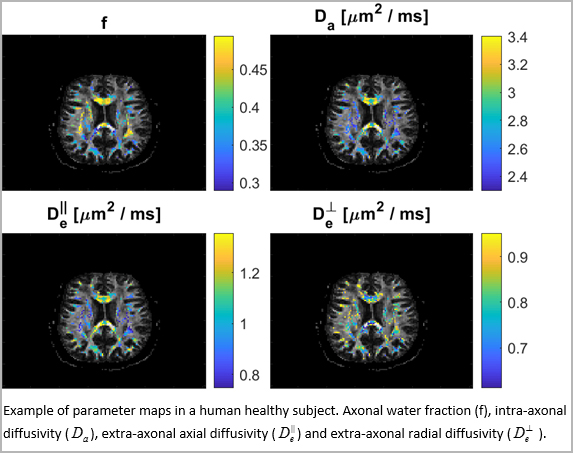
Our research group subsequently developed a fast MR method that allows healthy subjects to be examined by a diffusion weighted imaging sequence that is sensitive to brain microstructure (Hansen 2013 & 2016). It was later combined with biophysical modeling to provide clinically feasible maps of microstructural parameters (Hansen 2017 & Jespersen 2018).
 This sequence has proven sensitive to microstructural features of brain tumors (Tietze 2015) and subtle changes in brain tissue after concussions (Næss-Schmidt 2017). By validating the sensitivity of our methods to specific microctructural changes in animals expressing characteristics of neurological and psychiatric disorders (Chuhutin 2020, Khan 2018), we hope to improve our understanding of their disease mechanisms and to be able to better monitor the effect of disease modifying therapies.
This sequence has proven sensitive to microstructural features of brain tumors (Tietze 2015) and subtle changes in brain tissue after concussions (Næss-Schmidt 2017). By validating the sensitivity of our methods to specific microctructural changes in animals expressing characteristics of neurological and psychiatric disorders (Chuhutin 2020, Khan 2018), we hope to improve our understanding of their disease mechanisms and to be able to better monitor the effect of disease modifying therapies.
Currently, our work focuses on modeling the sensitivity of susceptibility contrast to microscopic tissue features and on high-field in vivo MRI as a way to unravel disease mechanisms.
References
- Chuhutin A, Hansen B, Wlodarczyk A, Owens T, Shemesh N, Jespersen SN. Diffusion Kurtosis Imaging maps neural damage in the EAE model of multiple sclerosis. NeuroImage. 2020;208:116406. doi: 10.1016/j.neuroimage.2019.116406.
- Khan AR, Hansen B, Wiborg O, Kroenke CD, Jespersen SN. Diffusion MRI and MR spectroscopy reveal microstructural and metabolic brain alterations in chronic mild stress exposed rats: A CMS recovery study. NeuroImage. 2018 Feb 15;167:342-353. doi: 10.1016/j.neuroimage.2017.11.053. Epub 2017 Nov 28.
- Næss-Schmidt ET, Blicher JU, Eskildsen SF, Tietze A, Hansen B, Stubbs PW, Jespersen S, Østergaard L, Nielsen JF. Microstructural changes in the thalamus after mild traumatic brain injury: A longitudinal diffusion and mean kurtosis tensor MRI study. Brain Inj. 2017;31(2):230-236. doi: 10.1080/02699052.2016.1229034.
- Tietze A, Hansen MB, Østergaard L, Jespersen SN, Sangill R, Lund TE, Geneser M, Hjelm M, Hansen B. Mean Diffusional Kurtosis in Patients with Glioma: Initial Results with a Fast Imaging Method in a Clinical Setting. AJNR Am J Neuroradiol. 2015;36(8):1472-8. doi: 10.3174/ajnr.A4311.
- Hansen B, Lund TE, Sangill R, Jespersen SN. Experimentally and computationally fast method for estimation of a mean kurtosis. Magn Reson Med. 2013;69(6):1754-60. doi: 10.1002/mrm.24743.
- Hansen B, Shemesh N, Jespersen SN. Fast imaging of mean, axial and radial diffusion kurtosis. NeuroImage 2016:142:381-393 doi: 10.1016/j.neuroimage.2016.08.022.
- Hansen B, Khan AR, Shemesh N, Lund TE, Sangill R, Eskildsen SF, Ostergaard L, Jespersen SN. White matter biomarkers from fast protocols using axially symmetric diffusion kurtosis imaging. NMR Biomed, 2017; 30: e3741 doi: 10.1002/nbm.3741
- Jespersen SN, Olsen JL, Hansen B, Shemesh N. Diffusion time dependence of microstructural parameters in fixed spinal cord. NeuroImage 2018:182:329-342 doi: 10.1016/j.neuroimage.2017.08.039
- Jespersen SN, Bjarkam CR, Nyengaard JR, Chakravarty MM, Hansen B, Vosegaard T, Østergaard L, Yablonskiy D, Nielsen NC, Vestergaard-Poulsen P. Neurite density from magnetic resonance diffusion measurements at ultrahigh field: comparison with light microscopy and electron microscopy. NeuroImage. 2010; 49(1):205-16. doi: 10.1016/j.neuroimage.2009.08.053.
Contacts
Professor Sune Nørhøj Jespersen, e-mail: sune@cfin.au.dk
Associate professor Brian Hansen, e-mail: brian@cfin.au.dk