Ongoing projects
This study aims to understand the early stages of Parkinson's by using multiple imaging techniques like MRI and PET scans of individuals at high risk, mainly in patients with isolated rapid eye movement sleep behavior disorder (iRBD). The goal is to identify early signs of disease progression and understand how different brain regions are affected over time.
We hypothesize that pathological processes in Parkinson's are temporally related and coexist in multiple brain areas during the prodromal stage. By using state-of-the-art imaging and statistical methods, we aim to elucidate these relationships and potentially identify patterns that can predict the progression to Parkinson's.
The project involves studying MRI and PET scans to detect changes in brain blood flow, neuroinflammation, dopaminergic and cholinergic functions in high-risk individuals over a three-year period. By combining data from different imaging techniques and long-term clinical follow-up, we aim to understand the relationships between various brain changes and predict the progression to Parkinson's and related diseases.
The findings of this study could lead to the discovery of early progression-biomarkers of iRBD patients and help predict clinical progression and identify new treatment targets for Parkinson's.
Contact

Andreas Baun, PhD student
Supervisor: Nicola Pavese, MD, Prof.
Humans maintain steady physiological conditions through dynamic, self-regulating multi-organ interactions with energy-consuming systemic feedback loops that orchestrate organs to respond to perturbations. This is known as homeostasis. Dynamic whole-body PET/CT imaging has the potential to detect deviations in the balanced metabolic state using the radioactive glucose analogue 18F-FDG.
This study aims to explore variations in the inter-organ glucometabolic correlation in diabetes and lymphoma patients in comparison to a healthy control group. Applying correlation analyses and metabolic connectivity frameworks, metabolic alterations caused by diabetes and lymphoma can be quantified, making it possible to evaluate if the alterations can be indicative of the diseases and if the effects can be separated on a group-level and individual level.
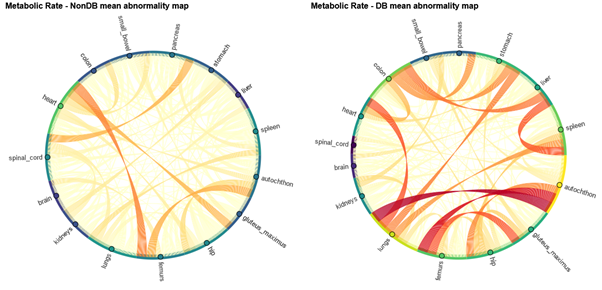
Figure 1: Group abnormality maps created with connectome analysis from a group of patients without diabetes (left) and a group of diabetes patients (right) when compared to a control group of patients without diabetes
Key Research Methods:
- Organ glucose uptake measured with [18F]-FDG whole-body dynamic PET
- Connectome analysis using inter-organ correlations
Contact

Marius Eldevik Rusaas, PhD student
Main Supervisor: Ole Lajord Munk, PhD, Professor
This PhD project aims to describe the underlying patterns of neurodegeneration in early and prodromal Lewy body disease (LBD) by utilizing an imaging battery, polysomnography and a variety of clinical measures. We have followed up our existing cohort of patients with idiopathic Parkinsons disease (iPD) and isolated REM behaviour disorder (iRBD), and one of the hypotheses were that iRBD patients upon follow-up will follow the proposed body-first trajectory towards the group of iPD patients with premotor RBD (paper 1).
Secondly, patients with the rare condition called pure autonomic failure (PAF) were hypothesized to be a very early body-first phenotype with markedly more pronounced peripheral involvement, but still with early stages of body-first LBD and progression patterns in line with the body-first (“bottom-up”) spreading of the disease (paper 2).
By studying novo GBA-PD and comparing these to iPD patients we aimed to investigate whether GBA-PD is predominantly body-first PD, and we included non-manifesting carriers of GBA-mutations to see if these have signs of early body-first pathology in the periphery such as abnormal cardiac MIBG (paper 3).
Contact

Casper Skjærbæk, PhD student
Main supervisor: Per Borghammer, PhD, Clinical Professor
Lewy Body Disorders (LBD), including Parkinson’s disease (PD) and dementia with Lewy bodies (DLB), are characterized by the pathological spread of alpha-synuclein (α-syn) along the gut–brain axis. In most DLB cases, Alzheimer’s disease (AD) pathology is also present, and conversely, a large fraction of AD cases exhibit α-syn co-pathology. This overlap creates diagnostic challenges because of the heterogeneous and overlapping clinical features.
This PhD project aims to establish a novel disease model that mimics the mixed and multi-system nature of DLB. By injecting α-syn fibrils into the gut of transgenic AD rats, we aim to reproduce early non-motor symptoms such as autonomic dysfunction and cognitive changes, as well as the characteristic mixed proteinopathies observed in human patients.
The model will help us explore how α-syn, tau and beta-amyloid co-pathologies influence disease progression and how they contribute to both motor and non-motor symptoms. Another important goal is to identify disease-specific protein strains in peripheral tissues as potential biomarkers for distinguishing DLB from AD and PD with dementia in early stages.
Key research methods
- Stereotactic surgery
- Behavioral symptom scoring
- Micro-PET imaging of gut, heart, and brain
- Immunohistochemistry
- Protein strain characterization with conformation-specific ligands
- Cryo-electron tomography
Contact

Contact Vasileios Theologidis, PhD student
Main supervisor: Nathalie Van Den Berge, PhD, Associate Professor
Parkinson’s Disease (PD) is a heterogeneous disorder with diverse motor and non-motor symptoms. This PhD project aims to develop magnetic resonance imaging (MRI)-based fingerprints for subtyping of PD from a deeply phenotyped cohort of patients to study markers of disease progression in different subtypes. The brain-first vs. body-first (BvB) model proposes two distinct trajectories of progression: pathology beginning in the gut/body and spreading bilaterally via peripheral nerves to the brain (body-first), or pathology beginning unilaterally in the amygdala or olfactory bulb and spreading to the body (brain-first).
This project consists of four interconnected studies aimed at refining PD subtyping. The first study investigates neurodegeneration gradients and hemispheric asymmetry, testing whether body-first PD follows a bottom-up pattern of atrophy in the brainstem and basal forebrain, while brain-first PD exhibits a top-down trajectory. The second study focuses on nigrostriatal pathway degeneration, utilizing MRI and PET imaging to assess how pathology spreads within the substantia nigra and striatum. In brain-first PD, degeneration is expected to progress from the lateral to the medial substantia nigra, whereas body-first PD is hypothesized to exhibit more uniform degeneration. The third study explores the impact of Alzheimer’s disease (AD) co-pathology by incorporating plasma biomarkers (p-tau181, p-Aβ42/40) to distinguish pure PD subtypes from cases with overlapping AD pathology. Finally, the fourth study employs unsupervised machine learning to analyze multimodal data, including MRI, PET, autonomic dysfunction, sleep metrics, and biochemical markers, to identify PD subtypes and their distinct MRI-based signatures. Through these studies, this project aims to establish biologically grounded, globally accessible, and affordable MRI biomarkers for PD subtyping.
The project will leverage a cohort of ~400 PD patients and matched controls with multimodal imaging and longitudinal follow-ups (3-6 years). Structural MRI, neuromelanin MRI and diffusion MRI scans will be subjected to a range of simple-to-advanced MRI techniques, including PET-MR co-registration studies, voxel-based morphometric analyses and tractography. Statistical approaches include machine learning-based clustering, and progression modelling to identify robust subtypes of Parkinson’s Disease both with and without reference to the brain-versus-body-first theoretical disease model.
Contact

Anushree Krishnamurthy, PhD student
Main supervisor: Per Borghammer, MD, PhD, Professor
PET/CT is widely used in oncology to provide insight into tissue metabolism, for example, glucose metabolism. Conventional clinical PET/CT, however, typically yields only a single static image at one time point, which limits the precision with which results can be interpreted. Dynamic whole-body PET/CT allows the temporal behaviour of radiotracers to be followed throughout the body, enabling the derivation of quantitative measures of tissue function, e.g. distribution volume and metabolic rate.
This project aims to optimise parametric PET imaging and to assess its feasibility for routine clinical use. By developing and evaluating new kinetic models, the study investigates whether more accurate quantitative measures can enhance diagnostic accuracy in patients with cancer and other diseases. In addition, strategies for correcting patient motion during scanning are explored to determine their impact on image quality and robustness of quantification.
Key Research Methods
- Dynamic whole-body PET/CT with [18F]-FDG and PSMA tracers
- Application and comparison of reversible and irreversible kinetic models
- Motion correction of dynamic PET data
Contact

Josefine Rosenskjold Madsen, PhD student
Main supervisor: Ole Lajord Munk, PhD, Professor
This study aims to assess the effect of Spinal Cord Stimulation (SCS) on the cholinergic nervous system and the glucose metabolism in the brain of patients with Parkinson’s disease (PD) and gait problems. Furthermore, this is a feasibility study that investigates the safety of this treatment and the effect on clinical gait performance of the participants.
Gait problems are common in PD and are often resistant to other treatments, including medicine. SCS has been found to improve gait, such as freezing of gait, in a small number of patients with PD. The mechanism of action is, however, unclear, and the treatment does not seem to work well in all candidates. Recently it has been implicated that cholinergic dysfunction in PD caused by degeneration of brainstem locomotor regions (such as the pedunculopontine nucleus) is involved in the control of movement initiation and body equilibrium. The cholinergic PET tracer 18FEOBV targets the vesicular acetylcholine transporter which is specific to cholinergic pre-synaptic terminals. 18F-deoxyglucose (18F-FDG) PET is a marker of regional cerebral glucose metabolic rate (by the marker of synaptic activity).
With this double-blind, randomised, placebo-controlled study, we aim to shed light on the mechanism of action of SCS with 18FEOBV and 18F-FDG PET scans. We also aim to identify imaging biomarkers at baseline that could be predictive of a favourable or a negative outcome of SCS and improve patient selection.
Contact

Miriam Højholt Terkelsen, MD, PhD student
Main supervisor: Nicola Pavese, MD, Prof.
Time-Restricted Eating (TRE 18:6) may improve cardiometabolic health, but benefits are rarely reflected in outcomes such as body weight and HbA1c. Obesity and cardiovascular disease are major public health challenges; prediabetes is associated with endothelial dysfunction, coronary microvascular dysfunction, and reduced myocardial flow reserve (MFR).
We will investigate whether 3 months of TRE improves MFR, organ-specific insulin sensitivity, and metabolic flexibility compared with a standard diet (SDD) in a randomized, controlled crossover study in overweight individuals with prediabetes. The primary endpoint is MFR; secondary endpoints include organ-specific insulin sensitivity, organ-specific glucose, fatty acid, and ketone metabolism. Exploratory outcomes are autonomic nervous system function assessed by heart rate variability (HRV) and brain derived neurotrophic factor (BDNF).
We employ whole-body PET/CT with [15O]H₂O (perfusion studies – MFR), [18F]FDG (insulin-stimulated glucose uptake), [11C]palmitate (fatty acid metabolism), and [11C]β-hydroxybutyrate (ketone uptake) combined with a hyperinsulinemic–euglycemic clamp, biopsies, and MRI (liver/muscle). To account for changes in behaviour during intervention periods, participants will perform daily home measurements: smart scale, smartwatch (activity and heart rate), instructions to walk 6–10,000 steps/day, and photoplethysmography-based nighttime HRV. In the week preceding study day, 24-hour blood pressure and Holter ECG (for HRV measurement) will be conducted. Participants will also measure home blood pressure weekly and weigh themselves daily.
We expect a 20% increase in MFR, improved insulin sensitivity, greater ketone/fatty acid utilization, and higher parasympathetic tone (assessed as an increase in HRV) following TRE. This project builds on data from a randomized crossover trial with alternate-day fasting over 3 weeks (36:12) vs. SDD, where a 34% increase in MFR was observed—effects typically only seen after long-term statin or beta-blocker therapy. TRE as a dietary strategy is feasible over extended periods and holds potential for significant clinical impact.
The prevalence of prediabetes and overweight is rising, and sustained lifestyle changes are difficult to achieve. TRE is simple, no-cost, and easily implementable. Organ perfusion and metabolism respond rapidly and are closely linked to cardiovascular event risk. The mechanism behind MFR improvement is unknown; our design can test whether increased parasympathetic tone, reduced blood pressure, and a shift in substrate utilization contribute to mediating these effects.
Contact

Asger Maare Søndergaard, PhD student
Main supervisor: Lars Christian Gormsen, Clinical Professor, Department Chair
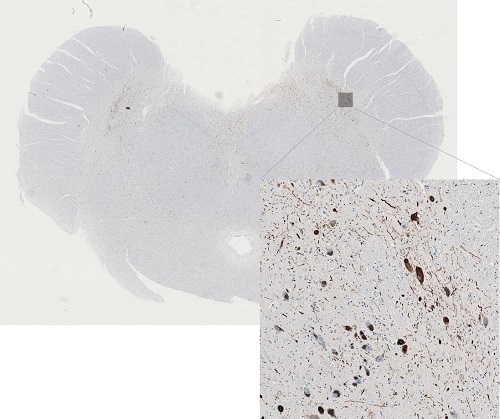
This PhD-study investigates the distribution and burden of alpha-synuclein pathology in post-mortem human brains from patients with Parkinson’s disease (PD) and incidental Lewy body disease using immunohistochemical (IHC) methods. The post-mortem brains used in this study was collected in 1945-1982 and are part of the Danish Brain Collection (previously located in Risskov).
Neuropathological studies have shown a brainstem-predominant pattern of alpha-synuclein pathology and a limbic system-predominant pattern. Brains from the Danish Brain Collection have both hemispheres conserved in a fixative making it possible to perform bilateral IHC analyses.
We aim to explore the symmetry/asymmetry aspect of PD by investigating the alpha-synuclein distribution bilaterally.
Here, we hypothesize that brainstem-predominant cases will show a symmetrical spreading pattern representing PD cases, where pathology originates in the autonomic nervous system, whereas limbic system-predominant cases will show an asymmetrical spreading pattern representing PD cases, where pathology originates within the brain in a single site, i.e. unilaterally.
Contact

Contact Mie Just, PhD student
Main supervisor: Per Borghammer, PhD, Clinical Professor