Image Modelling and Dementia research
Our research group develop image processing tools that allow us to combine images from groups of patients to understand disease processes and quantify progression in various brain disorders. We have a particular focus on Alzheimer’s Disease and combine various disease markers developed by our colleagues at the Neuroradiology Research Unit, the PET Center, Department of Nuclear Medicine, and collaborators abroad.
Our research? group develops methods to quantify brain changes based on MR images, providing tools to better understand normal brain development and the course of brain disorders such as Alzheimer’s Disease (AD). In particular, we have developed methods to extract the cerebral cortex from MR images of individual brains, using algorithms that include the delineation of the inner and outer boundaries of the cerebral cortex – See the figure below. With this technique, we and other researchers can characterize the cerebral cortex in great detail, quantifying its local thickness, surface area, and the extent to which it folds (gyrification), both in the developing and ageing brain, and in patients with neurodevelopmental, psychiatric, or neurodegenerative disorders.
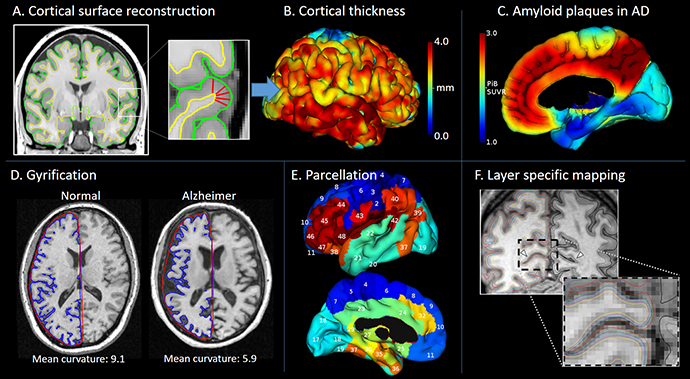
Panel A shows how the cerebral cortex can be reconstructed from MR images using 3D surface meshes that delineate its inner and outer boundaries, although image voxels are large relative to the thickness of the cortex. Panel B shows a color-coding of the cerebral cortex, indicating its local thickness. The surface meshes also permit cortical surface area to be calculated and the cortex folding to be quantified. Panel D shows how these measurements provide invaluable tools when investigating the developing and ageing brain as well as a range of diseases and neurodevelopmental disorders. Panel E shows how accurate identification of the cortical surface allows specific brain regions to be identified (parcellation) on the otherwise convoluted brain surface. This ‘map’ of the brain territories provides important reference points when reporting the results of other types of MR acquisitions (e.g. diffusion-, perfusion-, or susceptibility-weighted) or molecular images recorded by positron emission tomography (PET). Panel F shows how high resolution MR images can be used in the attempt to identify specific cell layers of the cortex (adapted from Polimeni et al.)
References
- Parbo P, Ismail R, Hansen KV, Amidi A, Mårup FH, Gottrup H, Brændgaard H, Eriksson BO, Eskildsen SF, Lund TE, Tietze A, Edison P, Pavese N, Stokholm MG, Borghammer P, Hinz R, Aanerud J, Brooks DJ. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer's disease. Brain. 2017;140(7):2002-2011. doi: 10.1093/brain/awx120.
- Eskildsen SF, Gyldensted L, Nagenthiraja K, Nielsen RB, Hansen MB, Dalby RB, Frandsen J, Rodell A, Gyldensted C, Jespersen SN, Lund TE, Mouridsen K, Brændgaard H, Østergaard L. Increased cortical capillary transit time heterogeneity in Alzheimer's disease: a DSC-MRI perfusion study. Neurobiol Aging. 2017 Feb;50:107-118. doi: 10.1016/j.neurobiolaging.2016.11.004.
- Næss-Schmidt E, Tietze A, Blicher JU, Petersen M, Mikkelsen IK, Coupé P, Manjón JV, Eskildsen SF. Automatic thalamus and hippocampus segmentation from MP2RAGE: comparison of publicly available methods and implications for DTI quantification. Int J Comput Assist Radiol Surg. 2016; 11(11):1979-1991. doi: 10.1007/s11548-016-1433-0.
- Eskildsen SF, Coupé P, García-Lorenzo D, Fonov V, Pruessner JC, Collins DL; Alzheimer's Disease Neuroimaging Initiative. Prediction of Alzheimer's disease in subjects with mild cognitive impairment from the ADNI cohort using patterns of cortical thinning. Neuroimage. 2013;65:511-21. doi: 10.1016/j.neuroimage.2012.09.058.
- Eskildsen SF, Coupé P, Fonov V, Manjón JV, Leung KK, Guizard N, Wassef SN, Østergaard LR, Collins DL; Alzheimer's Disease Neuroimaging Initiative. BEaST: brain extraction based on nonlocal segmentation technique. Neuroimage. 2012; 59(3):2362-73. doi: 10.1016/j.neuroimage.2011.09.012.
Contact
Associate professor Simon Fristed Eskildsen, e-mail: seskildsen@cfin.au.dk