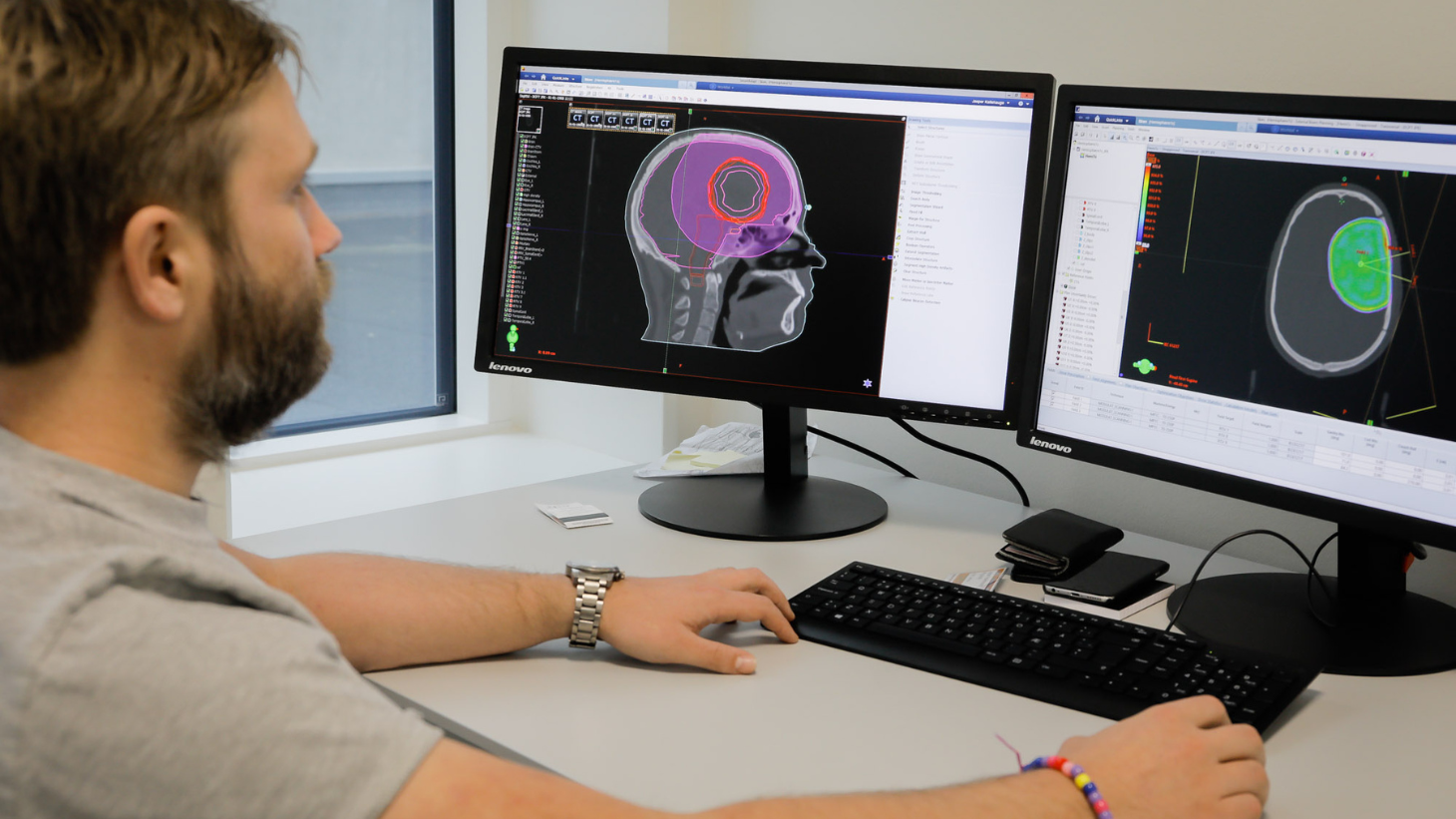
Medulloblastoma
HR-MB. SIOP High-Risk Medulloblastoma: An international prospective trial on high-risk Medulloblastoma in patients older than 3 years
An international study comparing a number of treatment options for patients older than three years with medulloblastoma and clinical or biological features that make these patients more difficult to treat (high-risk disease).
Ependymoma
SIOP EP II: An international clinical program for the diagnosis and treatment of children, adolescents and young adults with ependymoma
The overall aim of this project is to improve the outcome of patients diagnosed with ependymoma by improving and harmonising the staging and the standard of care of this patient population and to improve the investigators understanding of the underlying biology thereby informing future treatment. The programme will evaluate new strategies for diagnosis (centralized reviews of pathology and imaging) and new therapeutic strategies in order to develop treatment recommendations.
National investigator: Ines Kristensen
DCPT investigator: Yasmin Lassen
Wilms tumor
- UMBRELLA (UMBRELLA PROTOCOL SIOP-RTSG 2016): Integrated research and guidelines for standardized diagnostics and therapy
Rhabdomyosarcoma (RMS)
FaR-RMS. An overarching study for children and adults with Frontline and Relapsed RhabdoMyoSarcoma
The trial is trying to answer a number of questions about the treatment of RMS. These include looking at:
- new combinations of chemotherapy as initial treatment (induction)
- the timing, dose and extent of radiotherapy
- chemotherapy to keep the cancer under control after initial chemotherapy.
The main aims of the trial are to improve treatment outcomes and quality of life.
National investigator: Lisa Hjalgrim
DCPT investigator: Akmal Safwat
Neuroblastoma HR-NLB
Randomized, international and multicentric phase 3 study that
evaluates and compares 2 treatment strategies in 3 therapeutic
phases (induction, high-dose chemotherapy and radiotherapy) for
patients with high-risk neuroblastoma.
R-I:
Comparison of the EFS rate of 2 induction regimens, GPOH and
RAPID COJEC, in patients with high-risk neuroblastoma.
R-HDC:
Comparison of the EFS rate of single HDC with busulphan and
melphalan (Bu-Mel) versus tandem HDC with Thiotepa followed by
Bu-Mel in patients with high-risk neuroblastoma.
R-RTx:
Comparison of the EFS rate of 21.6 Gy radiotherapy to the preoperative tumor bed versus 21.6 Gy radiotherapy and a sequential
boost up to 36 Gy to the residual tumor in patients with macroscopic
residual disease after HDC and surgery.
National investigator: Jesper Sune Brok
DCPT investigator: Britta Weber
Atypical teratoid/rhabdoid tumours: AT-RT-01
Prospective, open label, multicentre, international umbrella trial including a randomized phase III study evaluating the non-inferiority of 3 courses of high-dose chemotherapy compared to focal radiotherapy plus standard chemotherapy as a consolidation measure following conventional chemotherapy in children with ATRT ranging from 12 – 35 months at the time of consolidation (RT vs. HDCT).
National investigator: Karsten Nysom
DCPT investigator: Yasmin Lassen
All paediatric cancers
HARMONIC: Health Effects of CArdiac FluoRoscopy and MOderN RadIotherapy in PediatriCs
The goal of the HARMONIC-RT study is to evaluate late health and social outcomes of modern external beam radiotherapy techniques in paediatric patients, based on the setting-up of a European, long-term registry complemented by a biobank.
National investigator: Yasmin Lassen
DCPT investigator: Yasmin Lassen