Computational medical physics: Target definition and treatment planning
We use machine learning, data mining and inverse optimisation to develop novel treatment planning methods for radiotherapy.
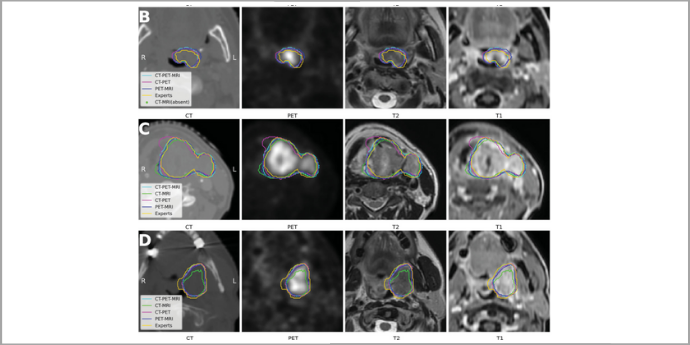
Figure: Three selected patient’s original CT, PET, MRI (T2, T1) images, deep learning predicted contours are compared from different image combinations. (B): GTV size is 5.6 cm3 , a case with small tumor size. (C): GTV size is 54.1 cm3 , a large tumor case. (D): GTV size is 35.5 cm3 , a case with planning CT artifacts.
We use computational methods such as machine learning, data mining and inverse optimisation, to challenge existing paradigms in radiotherapy for development of novel treatment planning methods. We use advanced analysis of medical images including CT, PET and MR scans, in large patient cohorts, with focus on head and neck cancer and breast cancer. We specifically address the problems that give rise to the largest sources of uncertainty in radiotherapy planning; namely target definition, dose optimisation, and anatomical changes during treatment.
Target delineation is a time-consuming process, which is also prone to large uncertainties and subjective assessment by the delineating clinician. We use deep learning to automatically segment the gross tumour volume in multimodality images, for use in interactive delineation tools. By introducing a deep learning generated segmentation in an interactive user interface, we aim to speed up the delineation process while at the same time reducing the uncertainty related to the delineation for a more accurate treatment.
We investigate the implementation of risk models for tumour spread, which can be used for probabilistic optimisation of dose distribution around the tumour. This approach will give the potential to reduce high radiation doses to volumes of tissue where the risk of finding cancer cells is low, and hence reduce the risk of side effects.
Other projects include individualized robust optimization to address anatomical changes during treatment, data mining for modelling patient individual risk factors, and development of automated treatment planning methods.
Overall, we aim to enhance tailoring of radiotherapy to the individual patient through adaptation to patient specific risk factors from medical images. Moreover, we expect that the introduction of automated and computational methods will lead to an overall decreased level of uncertainty in the treatment and increased treatment quality at both individual and population basis.
People
 Professor of medical physics
Professor of medical physics
Stine Sofia Korreman
stkorr@rm.dk
Further information.
 Postdoc
Postdoc
Camilla Skinnerup Byskov
CAMBYS@rm.dk.
Further information.
Postdoc
Jintao Ren
JINREN@rm.dk
PURE, Aarhus University. Further information.
Postdoc
Lasse Hindhede Refsgaard
LASREF@rm.dk.
PURE, Aarhus University. Further information.
PhD student
Emma Skarsø Buhl
EMSKAR@rm.dk
Further information.
 PhD student
PhD student
Mathis Ersted Rasmussen
mathis.rasmussen@rm.dk
PURE, Aarhus University.
 PhD student
PhD student
Nadine Vatterodt
nadine.vatterodt@clin.au.dk
 PhD student
PhD student
Kristoffer Moos
KRIMOO@rm.dk
PhD student
Ihsan Bahij
ihsan.bahij@rm.dk
Collaborating researchers
 Associate professor of medical physics
Associate professor of medical physics
Jasper Albertus Nijkamp
jaspernijkamp@clin.au.dk
Further information.
 Consultant, Associate Professor of Clinical Medicine
Consultant, Associate Professor of Clinical Medicine
Kenneth Jensen
kenneth.jensen@auh.rm.dk
Further information.
Associate professor
Jesper Folsted Kallehauge
jespkall@rm.dk
Further information.
PhD student
Zixiang Wei
ZIXWEI@rm.dk
PURE, Aarhus University.
Medical physicist, PhD
Ulrik Vindelev Elstrøm
ulrik.vindelev.elstroem@auh.rm.dk
PURE, Aarhus University.
Medical Physicist, Dep. of Oncology, Aarhus University Hospital
Anne Ivalu Sander Holm
annivaho@rm.dk
Professor, MD, Dep of Oncology, Aarhus University Hospital
Jesper Grau Eriksen
jesper@oncology.au.dk
Professor, MD, Dep. of Oncology, Aarhus University Hospital
Birgitte Offersen
Birgitte.Offersen@auh.rm.dk